A New Era for Cell Therapies
The recent clinical success of engineered T cells to treat cancer is creating palpable excitement and spurring numerous efforts to broaden their use for different cancer types and applications. But in spite of their potential to induce durable remission, cellular immunotherapies are not effective for everyone.
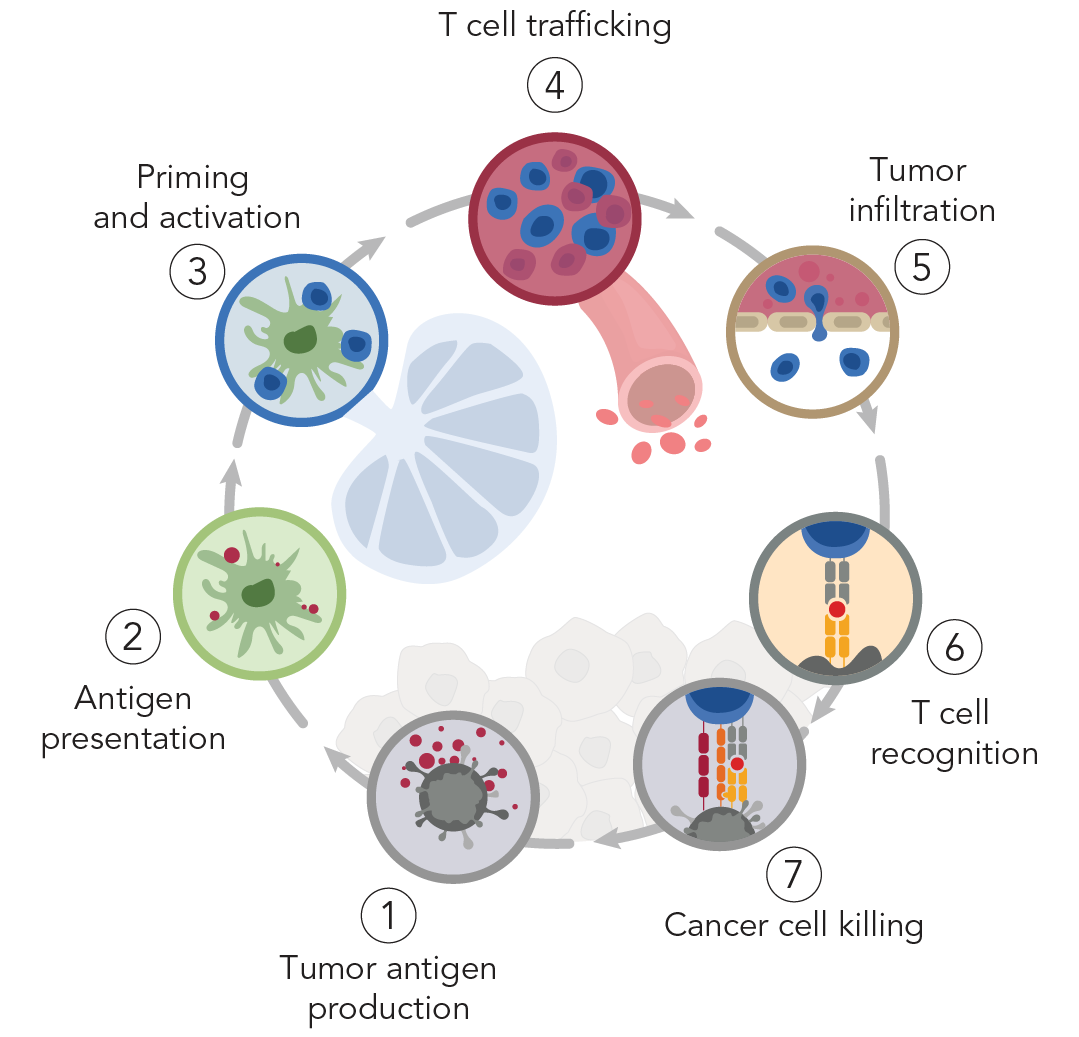
The factors that contribute to a poor response are multifaceted and include the degree to which key steps in the immunity cycle are effectively directed against tumors. These challenges include poor tumor infiltration, immune suppression, and lack of tumor-specific antigens.
Our research program draws from engineering disciplines – such as nanomaterials, scalable manufacturing, and drug delivery – in order to innovate new strategies to enhance T cell therapies and solve bottlenecks in the development pipeline that drive cost and limit broad access [1].
[1] “Synthetic immunity by remote control” Theranostics 10(8): 3652–3667 (2020).
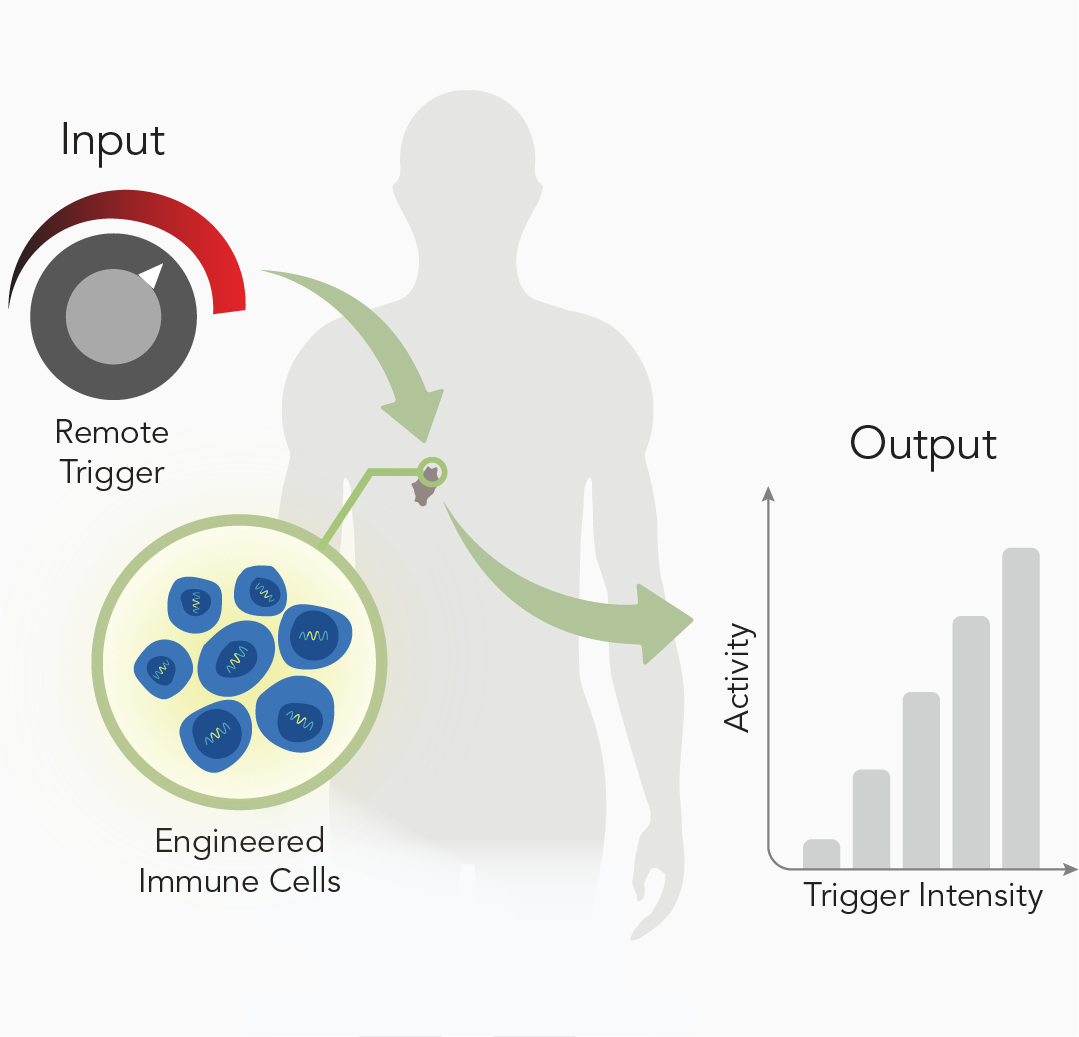
Remote Control
Our limited ability to control anti-tumor activity in tumors and at disease sites contributes to poor engineered T cell responses against solid malignancies. Current approaches rely on systemic co-delivery of immune-modulating adjuvants to support therapeutic T cell responses such as cell proliferation and tumor cell killing. However, these approaches lack precision and are associated with adverse effects including off-target killing and systemic toxicities. We are developing strategies for remote control of T cell responses – such as with heat pulses [2] to direct transcriptional profiles [3] – to increase the precision and enhance the efficacy of adoptive T cell therapies [4].
[2] “Remote control of mammalian cells with heat-triggered gene switches and photothermal pulse trains” ACS Synth. Biol. 7(4), 1167–1173 (2018).
[3] “Heat-triggered remote control of CRISPR-dCas9 for tunable transcriptional modulation” ACS Chem. Biol. 15(2), 533–542 (2020).
[4] “Enhanced intratumoural activity of CAR T cells engineered to produce immunomodulators under photothermal control” Nat. Biomed. Eng. 5, 1348–1359 (2021).
T Cell Delivery
The first FDA-approved CAR T-cell therapies, Kymriah™ (Novartis) and Yescarta™ (Kite), have demonstrated remarkable overall response rates (>80%) in treating B cell malignancies. However, T cell therapies are personalized medicines that require a bespoke development and manufacturing process that is costly and precludes broad use of T cell therapies as standard-of-care for cancer treatment. We are developing new engineering solutions – including scalable manufacturing [5] and non-viral gene delivery [6] – to lower costs, accelerate production, and make T cell therapies available to everyone.
[5] “Synthetic antigen-presenting cells for adoptive T cell therapy” Adv. Therap. 2100034 (2021).
[6] “In vivo mRNA delivery to virus-specific T cells by light-induced ligand exchange of MHC class I antigen-presenting nanoparticles” Sci. Adv. 8: eabm7950 (2022).