The Early Detection Problem
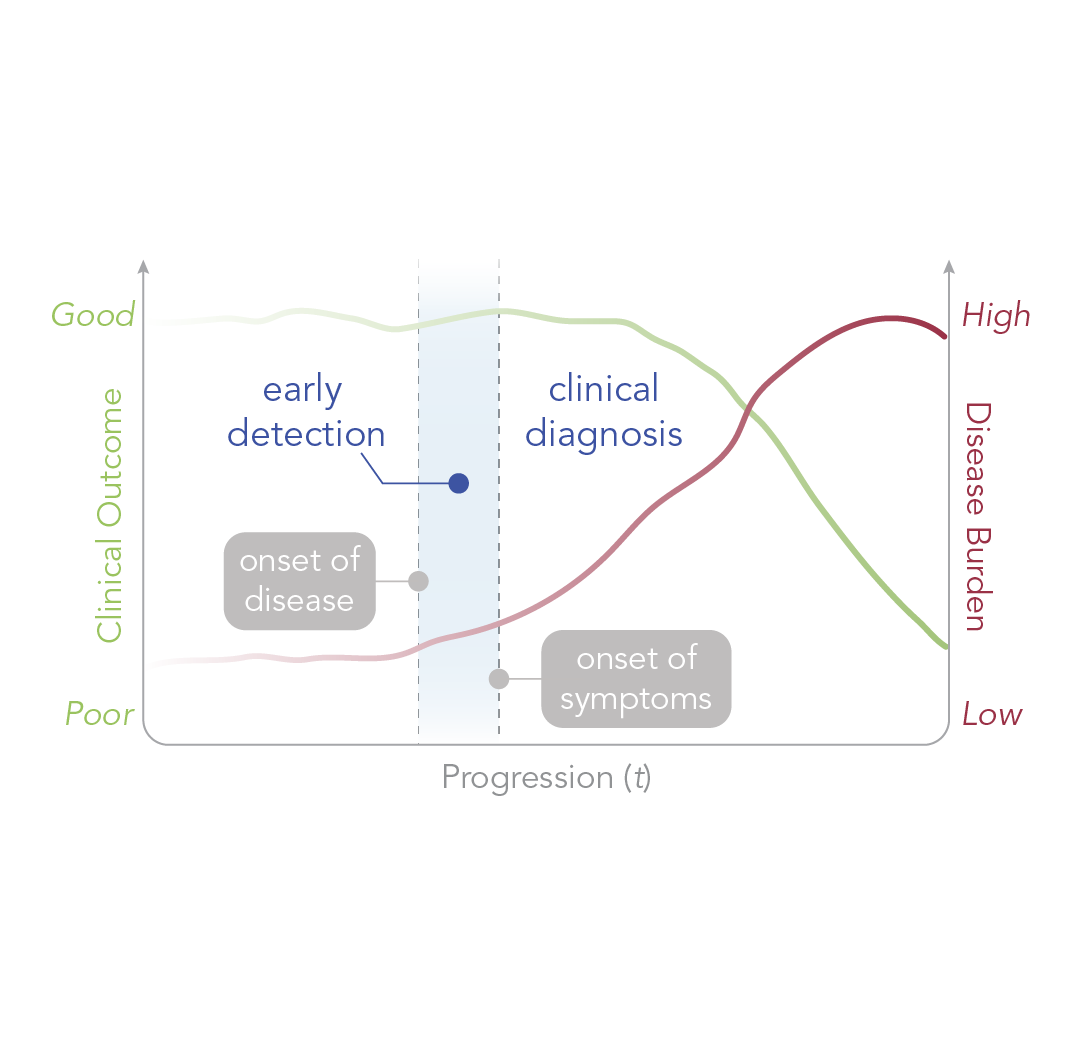
Intercepting disease early has an enormous impact on patient outcomes. For example in ovarian cancer, 5-year survival for stage I disease is over 90% compared to less than 20% at stage IV [1]. Despite this, disease is caught early only in a small segment of the population – even in those who are regularly screened.
Perhaps surprisingly, the underlying reasons that form this important problem are intuitive [2]. Early detection relies on screening blood for biomarkers, which are usually proteins or small molecules that are produced by diseased tissue. When these biomarkers are shed into blood, they are diluted by greater than 100,000-fold and circulate briefly before they are cleared.
The early detection problem, therefore, is one of biomarker scarcity. Without alternative strategies, there will continue to be a stark mismatch between the disease stage that could be detected by blood biomarkers and the stage that would best respond to treatment.
[1] “Cancer Facts and Figures 2019” American Cancer Society.
[2] “Mathematical framework for activity-based cancer biomarkers” Proc. Natl. Acad. Sci. USA 112(41), 12627–12632 (2015).
A Bioengineered Solution
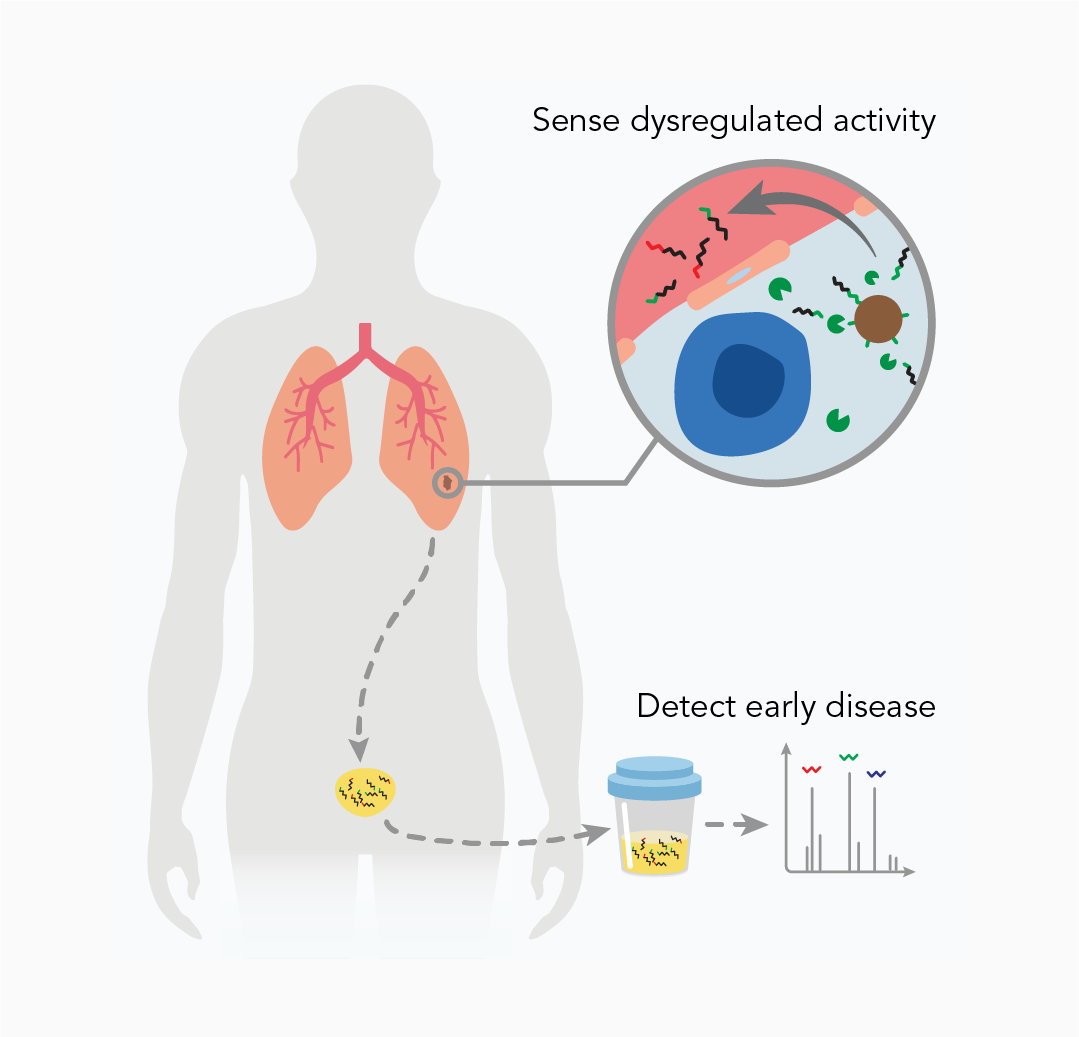
We are pioneering a new class of biological sensors called synthetic biomarkers to intercept early disease [3]. Unlike traditional diagnostics, our sensors are not built to analyze blood samples for scarce biomarkers. Rather, they are designed to be deployed in the body where they query target organs and amplify early disease signals before symptoms arise.
In a foundational study [4], we first reported the design of synthetic biomarkers that leverage the catalytic activity of dysregulated proteases to locally amplify signals at sites of disease, which are then naturally cleared into recipient urine for noninvasive detection. In mouse models of cancer, we showed that urinalysis allowed discrimination of small tumors with higher sensitivity compared to FDA-approved blood biomarkers.
Our approach is not limited for early detection as proteases are key players across broad biological pathways. Instead, synthetic biomarkers can be engineered to report on drug responses or anticipate emergence of resistance to help develop better therapies.
[3] “Synthetic Biomarkers: A 21st century path to early cancer detection” Nat Rev Cancer 21, 655–668, (2021).
[4] “Mass-encoded synthetic biomarkers for multiplexed urinary monitoring of disease” Nat Biotechnol 31, 63-70, (2013).
Broad Disease Applications
Transplant Rejection | Working with physicians at the Emory Transplant Center, we are developing immune sensors for noninvasive monitoring of allograft health. We recently showed that nanosensors that detect proteases produced by recipient T cells during the onset of acute cellular rejection (ACR) can serve as a noninvasive biomarker of graft rejection [5]. We are building on this study to resolve different mechanisms of anti-graft activity including antibody mediated rejection (AMR).
[5] “Non-invasive early detection of acute transplant rejection via nanosensors of granzyme B activity” Nat. Biomed. Eng. 3, 281–291 (2019).
Checkpoint Blockade Immunotherapy | Immune checkpoint blockade therapy (ICB) has transformed the treatment of cancer for patients across a broad range of malignancies. Yet despite treatment responses that are unprecedented and durable, the majority of patients do not experience a clinical benefit from treatment, and some responders relapse and acquire resistance. We are developing ImmuNe Sensors for monItorinG cHeckpoint blockade Therapy (INSIGHT) to predict treatment efficacy and indicate resistance to ICB therapy [5].
[6] “Urinary detection of early responses to checkpoint blockade and of resistance to it via protease-cleaved antibody-conjugated sensors” Nat. Biomed. Eng. 6, 310–324 (2022).